New Drug
GM-XANTHO
INDICATION
Atopic Dermatitis
MECHANISM
- Anti-inflammatory
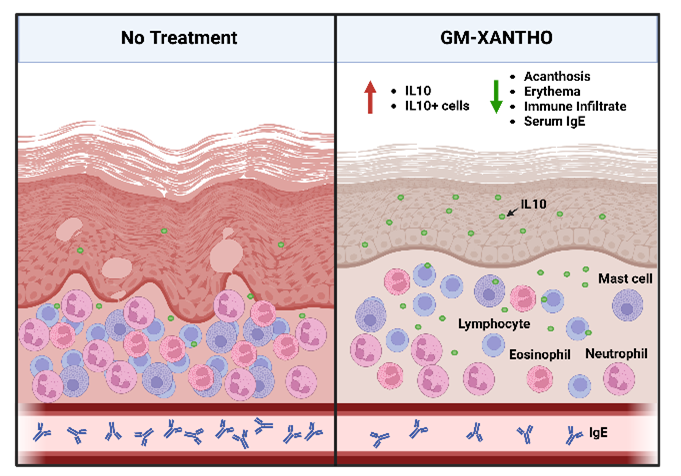
- To diminish the levels of pro-inflammatory cytokine RNA, pro-inflammatory immune cells, and IgE levels, concomitant with an augmentation in the levels of the anti-inflammatory cytokine IL-10 and IL-10-secreting cells.
STATUS
- Phase II
ADVANTAGES
- Botanical new drugs have high barriers to entry, and raw materials are difficult to obtain.
- The effect is as good as steroids but not recur in AD
- Safety and long-term use
MARKET
The Global Data estimates that in 2027, the sales were total $18.3B for atopic dermatitis worldwide.
- GM-XANTHO demonstrated the capacity to diminish the levels of pro-inflammatory cytokine RNA, pro-inflammatory immune cells, and IgE levels, concomitant with an augmentation in the levels of the anti-inflammatory cytokine IL-10 and IL-10-secreting cells. Consequently, this led to a reduction in epidermal thickness, immune infiltrate, and inflammatory responses, ultimately achieving therapeutic efficacy in the treatment of atopic dermatitis.
- GM-XANTHO has completed CMC, which has manufactured in accordance with PICS/GMP specifications. GM-XANTHO has completed pre-clinical pharmacology, pharmacokinetics and toxicity tests to confirm the efficacy of GM-XANTHO in the treatment of atopic dermatitis and has low side effects.