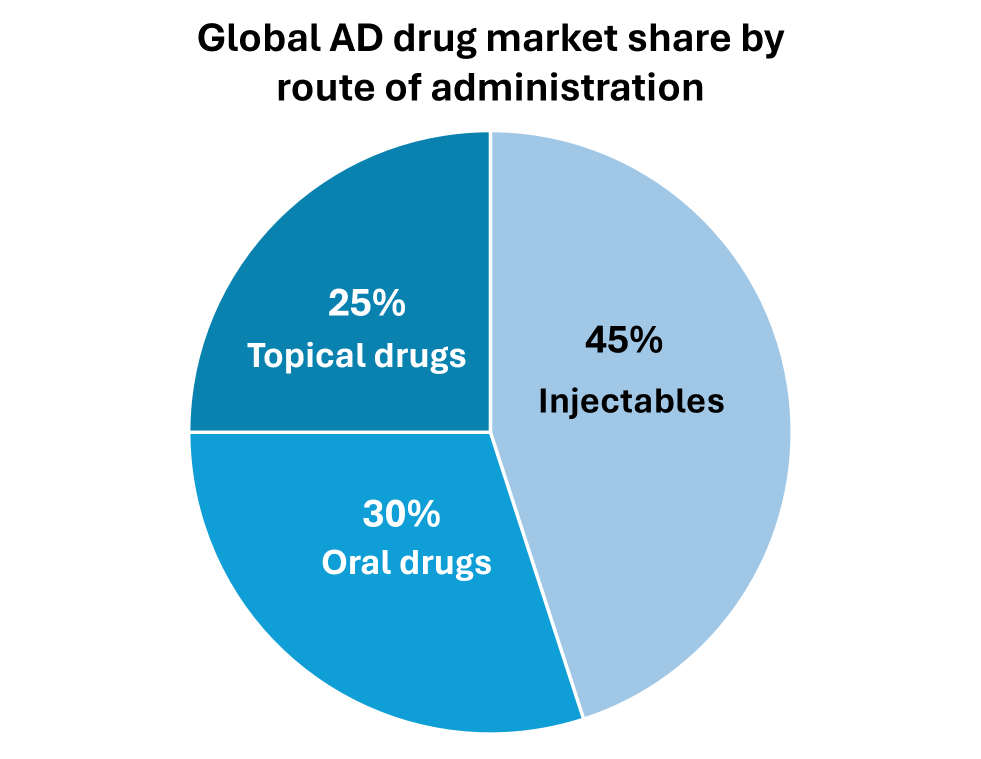
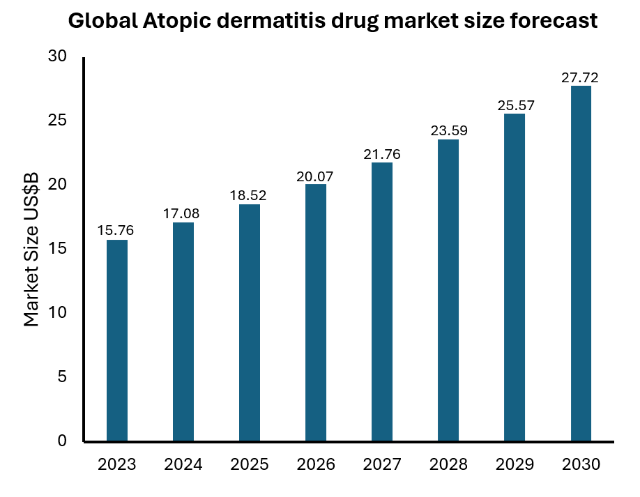
GM-XANTHO

**R&D Progress**
GM-XANTHO stands out as the world's only botanical drug for atopic dermatitis. Animal experiments have demonstrated its ability to induce the generation of the anti-inflammatory cytokine IL-10, thereby reducing skin inflammation. Simultaneously, it aids in repairing the skin barrier, alleviating itching, and lowering the concentration of IgE in the blood, achieving a comprehensive therapeutic effect.
GM-XANTHO obtained IND approvals for trials in Taiwan and the United States in November and December 2019, respectively, initiating Phase I/IIa trials. Following the completion of Phase I in Taiwan in March 2021, it swiftly entered Phase IIa trials, which concluded in February 2025. The Clinical Study Report (CSR) is expected to be available in Q2 2025.
**Introduction to Atopic dermatitis**
Atopic dermatitis is a chronic skin disease characterized by recurrent inflammation and itching, resulting from an imbalance in the skin's immune function. The inflammation and itching are caused by the immune dysfunction, leading to damage to the skin barrier through scratching. This damage results in increased inflammation, creating a vicious cycle that is challenging to heal. Atopic dermatitis remains an immunological disorder prone to relapses.
Current primary treatment options include topical corticosteroids, antihistamines, topical calcineurin inhibitors (TCI), immunosuppressants, ultraviolet light therapy, and biologics. Corticosteroids are presently the most effective and commonly used treatment method; however, their rapid and robust efficacy is often accompanied by significant side effects, such as skin atrophy, pigmentation changes (darkening or whitening), acne, and folliculitis. As a result, many individuals nowadays experience "corticosteroid phobia" and seek non-corticosteroid pharmacological treatments.
Antihistamines can help relieve itching but do not contribute to the etiological treatment. TCIs can only be used intermittently in the short or long term, with lingering safety concerns. Immunosuppressants like Janus kinase inhibitors (JAK inhibitors) provide rapid and effective relief, but their high cost and limited short-term use necessitate re-administration upon discontinuation, leading to potential relapses. Biologics are typically considered the last line of defense in treatment due to their high costs, requiring a minimum expenditure of over TWD 200,000 per course. While severe patients may experience significant improvement after a 16-week treatment, relapses are common, and resistance to the same drug may develop.
Therefore, there remains a substantial gap in the atopic dermatitis pharmaceutical market, and patients still require non-corticosteroid medications that are effective, suitable for long-term use, and capable of reducing the recurrence rate.