GM-XAN003

**R&D Progress**
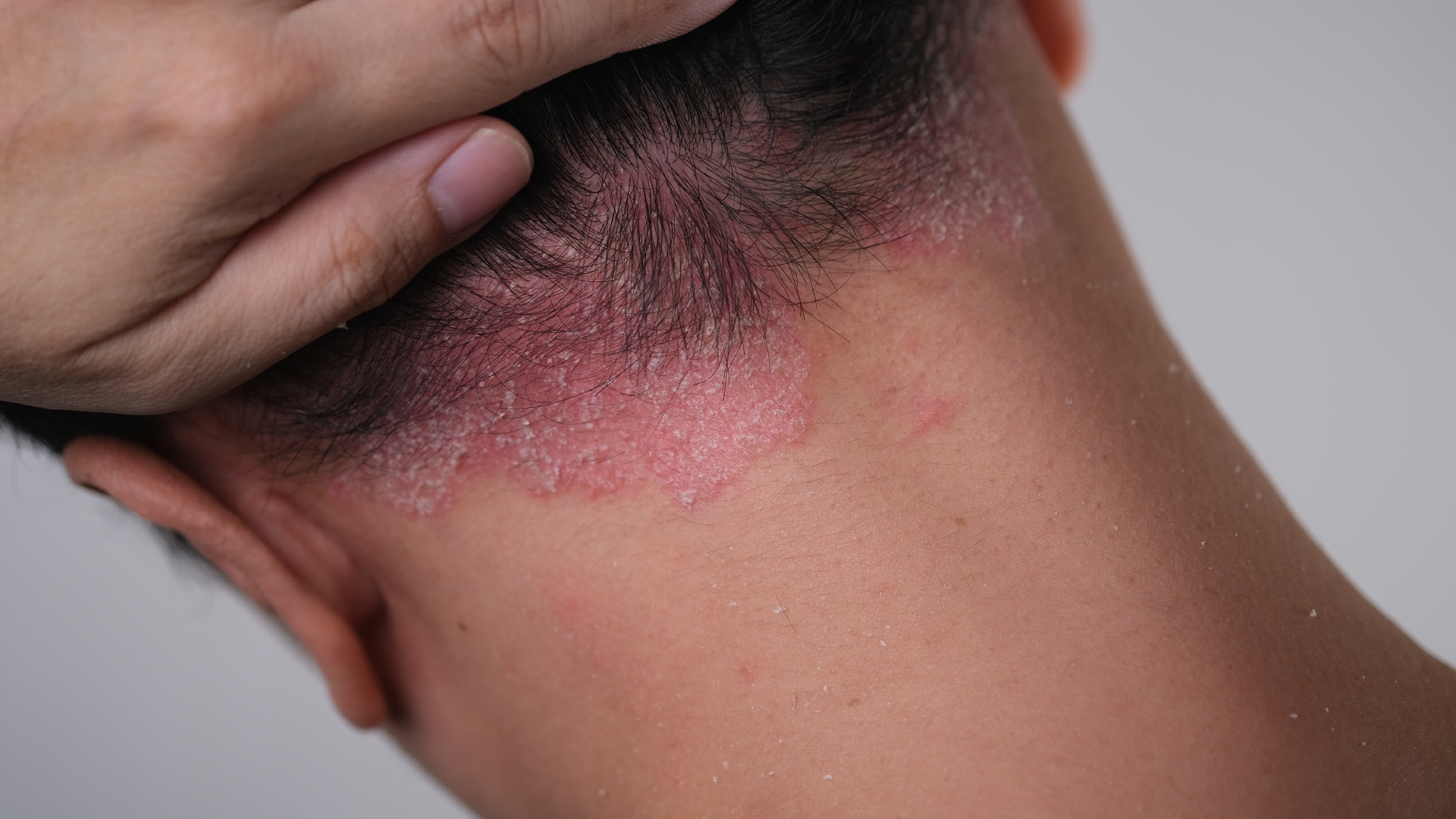
**Introduction to Plaque Psoriasis**

The primary frontline treatment in clinical practice includes topical medications such as steroids, vitamin D derivatives, and retinoids, with the addition of phototherapy or oral immune-modulating drugs like Janus kinase inhibitors (JAK inhibitors) based on the severity of the condition. Severe cases may also undergo antibody therapy with injectable biologics. However, the prevalent use of topical medications like steroids and vitamin D derivatives is hindered by their strong side effects, leading to reluctance among many patients. Moreover, clinical trials predominantly focus on therapies for moderate to severe cases, such as biologics or oral medications, resulting in fewer options for topical treatments tailored to mild cases. Therefore, there remains a need for topical medications with superior efficacy, minimal side effects, and suitability for long-term use for patients with mild symptoms.
**Market Overview**
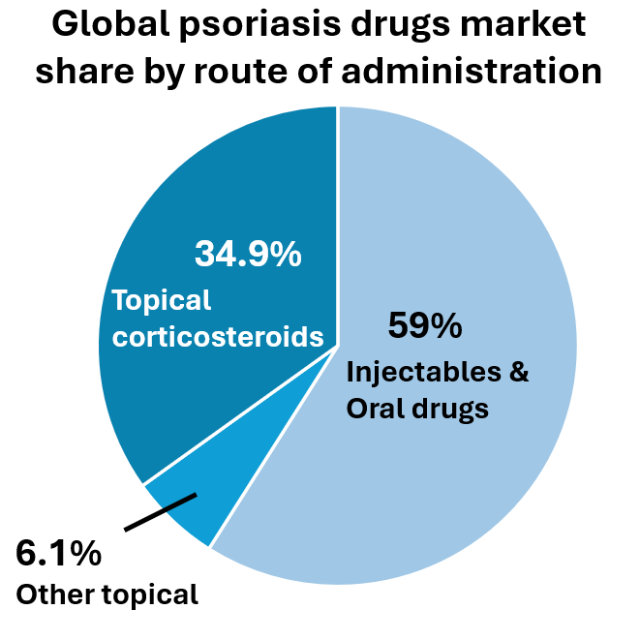
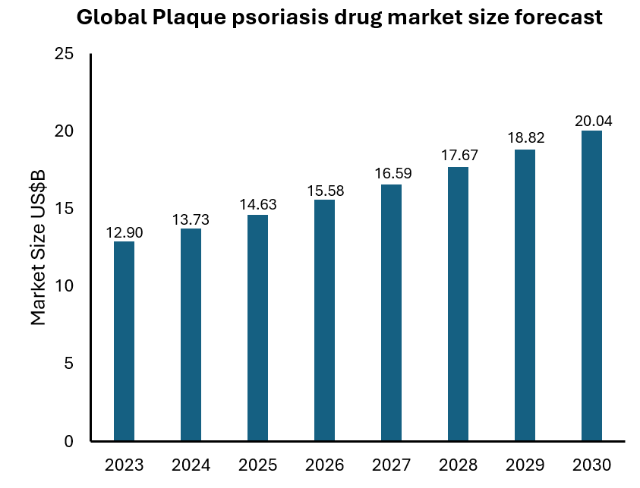
The global population of psoriasis is around 223 million with a prevalence rate of 2% -3%, and studies have shown that the global population of psoriasis is increasing. According to the market analysis report of Persistence market research, the global market size for psoriasis treatment was valued at USD 12.9 billion in 2023, and it is estimated to exceed USD 20 billion in 2030 at a compound annual growth rate (CAGR) of 6.5%. The topical drugs (including topical corticosteroids) segment accounted for 41% of the market share by route of administration in 2021 (Persistence market research 2022). The US is the largest market and accounted for 80% of the global sales for psoriasis medications, followed by Europe and Japan (Global Data 2020).